Written By Jim Burelbach, PhD, CCO, Fauske & Associates
The current pandemic due to SARS-CoV-2, the virus that causes COVID-19, has challenged us to re-think everyday activities. One critical aspect that is vital for a safe return to “normal” is an understanding of how SARS-CoV-2 is transmitted within a building or facility, i.e. airborne aerosol transmission. The below example illustrates how the FATETM software is easily applied to a building comprising interconnected, well-mixed regions. The results illustrate specific actions that can help minimize virus transmission by reducing the amount of virus aerosols within a building.
The FATE (Flow Aerosol Thermal and Explosion) Facility software, developed by Fauske & Associates, is a versatile tool for analyzing the transient behavior of facilities and processes during normal and off-normal conditions. It has been used in the chemical and nuclear industries to assess fire and smoke transport, hydrogen transport and accumulation, and to predict pressure and temperature behavior of nuclear waste packages. Learn more about these applications by downloading our whitepaper.
A unique feature of FATE is its ability to characterize and track aerosols. This capability lends itself to quantification of hazards from aerosol particulate released under off-normal process conditions. FATE models the transport of aerosol particles from an emission source through a facility and into the environment, accounting for any settling, impaction, or filtration which may occur. While it has historically been used to model industrial hazards (chemical and radiological) the software is general enough that it can track biological aerosols, such as SARS-CoV-2.
Multi-Region Example Model
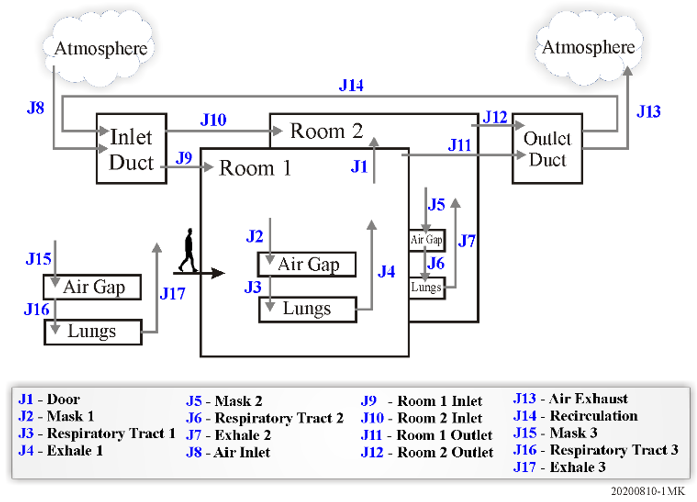
Consider the potential for virus aerosol transmission in an interconnected, multi-region facility with a shared ventilation system. An example model is illustrated in Figure 1. This example considers two rooms connected by a doorway and a shared ventilation system and includes features found in most ventilation systems which recondition, filter, and recycle the ventilation air. The aerosol source (an infected asymptomatic person) is treated as a constant rate emitter of aerosols developed through normal speech.
Within each room a healthy individual is represented by two regions (the air gap under a mask, if worn, and the lungs) and three junctions (the mask itself, the respiratory tract, and the exhale path). All virus aerosols that are inhaled are assumed to attach to the respiratory tract and pose a risk of infecting a healthy individual. A third individual is assumed to show up later and represents the possibility of virus transmission to someone who arrives in the building just after the other three individuals have left, but who can be exposed to a suspended aerosol that continues to be recirculated via the ventilation system.
The model uses published values for the airborne viral emission rate of SARS-CoV-2, measured droplet size and concentration in exhaled air representative of normal speaking, and the reported inhalation rate for light exercise. The model also considers deactivation of airborne virus based on published estimations of the half-life of SARS-CoV-2 in aerosols. The infectious dose for SARS-CoV-2 is currently not known and is estimated here based on a previous study related to SARS-CoV-1.
Figure 1 Multi-region model for airborne virus transmission
Example Results
The virus emitter (an infected person) and healthy Individual 1 are both in Room 1 (680 m3) for an eight-hour shift. A second, smaller room (136 m3) connects to Room 1 through a door. Individual 2 occupies Room 2. Both rooms share a ventilation system which provides four air changes per hour (ACH), with half of the air being recirculated. Four cases are analyzed:
1. with no ventilation and no masks,
2. with ventilation and no masks,
3. with a HEPA filter on the recirculated air and no masks, and
4. with both a HEPA filter and masks.
Figure 2 shows the distribution of aerosol mass for Case 2, with active ventilation but no masks. The aerosol removal rates by gravitational settling and ventilation to the environment are nearly identical. Aerosol removal by virus deactivation is significant, albeit smaller than by gravitational settling or ventilation. Although the aerosol source is in Room 1, the aerosols are transported to Room 2 by recirculated air through shared ventilation ducts, and Individual 2 in Room 2 receives appreciable dose.
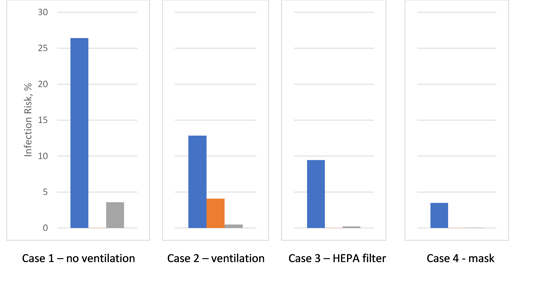
After the infected person leaves the building, the aerosol concentrations decay exponentially. Individual 3 enters Room 1 at 8 hr and is exposed to remaining virus aerosols, receiving a small but non-negligible dose. After 16 hours the three healthy individuals have accrued an infection risk of 12.9%, 4.1%, and 0.5%, respectively. Table 3 summarizes the results for all four cases. The effectiveness of various measures taken to reduce the virus transmission is illustrated in Figure 3. As expected, ventilation, with HEPA filters for recirculated air, and wearing masks reduce the infection risk. Without HEPA filters, recirculated air can spread virus aerosols to other rooms sharing the same ventilation system.
Figure 2 Time history of aerosol mass distribution for Case 2
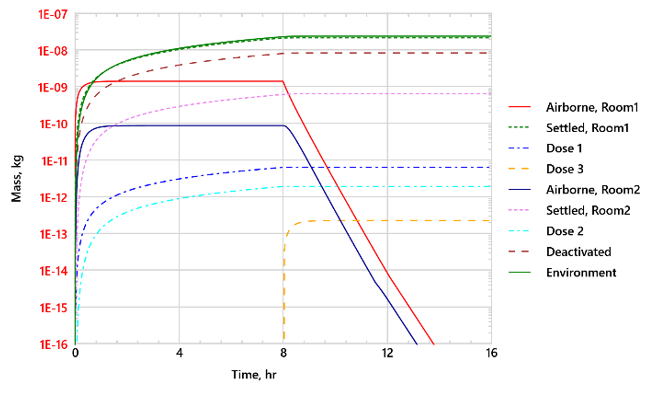
Table 1 Summary of dose and infection risk results
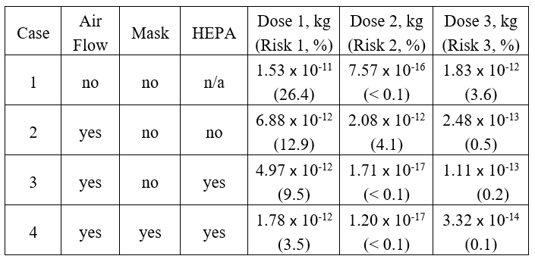
Figure 3 Infection risk (blue: Dose 1, orange: Dose 2, grey: Dose 3)
Conclusions
A mechanistic approach is presented to quantify airborne transmission and infection of SARS-CoV-2 using the facility modeling code FATE. Some key information about the pathogen such as the infectious dose of SARS-CoV-2 is not known, and the infection risks reported here are based on specific model assumptions. FATE can be used to quantify the effectiveness of various measures taken to reduce the airborne transmission of the virus as the model is easily modified to represent different building configurations and ventilation networks. Further technical details are provided by Kennedy et al. (2020)1.
The FATE model presented here demonstrates that for a multi-room facility, ventilation, with HEPA filters for recirculated air, together with wearing masks, reduces the infection risk. Without HEPA filters, the recirculated air can spread virus aerosols to other rooms sharing the same ventilation system. The FATE model is easily modified to accommodate alternate system design or performance characteristics to quantify the benefit of system modifications in reducing infection risk.
To read an extended version of this article please read our Summer 2020 Process Safety Newsletter. If you'd like to speak to a Fauske team member about the FATE software, feel free to contact us!
Sources:
1. Kennedy, M., Lee, S.J., and Epstein, M., 2020, “Modeling Aerosol Transmission of SARS-CoV-2 in a Multi-Room Facility,” submitted for publication in the Journal of Loss Prevention in the Process Industries.