How We Handle Reagents and Decomposition Products in Testing Activities
By: Charles Askonas, Chief Testing and Safety Engineer at Fauske & Associates, LLC
FAI Case Study of Dimethyl Sulfoxide (DMSO) Decomposition
 An important consideration in our testing business is knowing we can safely handle both the initial reagents and any post-test products of combustion or decomposition. The availability and use of appropriate Personal Protective Equipment (PPE) and engineering controls go a long way in the safe conduct of testing activities. However, a potentially overlooked but important consideration is to determine what the reaction products are before performing tests in order to properly handle them. Most of the time decomposition products are not fully known. However because of personnel safety and environmental considerations, we cannot take chances in our operations. While personnel safety is of course expected, our unique environmental consideration is the close proximity to the property line in the industrial park we are located in, as well as to a residential area. Therefore, our commitment to safe operations on a day-by-day basis has provided valuable experience to enable us to assess and handle new challenges presented by our customers’ samples.
An important consideration in our testing business is knowing we can safely handle both the initial reagents and any post-test products of combustion or decomposition. The availability and use of appropriate Personal Protective Equipment (PPE) and engineering controls go a long way in the safe conduct of testing activities. However, a potentially overlooked but important consideration is to determine what the reaction products are before performing tests in order to properly handle them. Most of the time decomposition products are not fully known. However because of personnel safety and environmental considerations, we cannot take chances in our operations. While personnel safety is of course expected, our unique environmental consideration is the close proximity to the property line in the industrial park we are located in, as well as to a residential area. Therefore, our commitment to safe operations on a day-by-day basis has provided valuable experience to enable us to assess and handle new challenges presented by our customers’ samples.
The ability to safely (and confidently) handle a reagent or reaction by-product depends on various properties including the volatility (e.g. normal boiling point), permissible exposure limit, specific toxic characteristics, and the ability to neutralize or destroy it by “scrubbing” using an appropriate solution. A few specific examples will be provided below.
While anhydrous hydrogen chloride is a toxic gas, it can be readily neutralized using sodium hydroxide solution. If it is used as a reagent, the lecture bottle must be equipped with a regulator to reduce the pressure to near atmospheric pressure in order to control the addition rate. Hydrogen chloride produced as a reaction product can be completely scrubbed in 10% sodium hydroxide solution. Workers working in a properly functioning fume hood wearing full facepiece cartridge respirators are adequately protected. The OSHA PEL is 5 PPM.
By comparison, reagents that are “slow scrubbers” with a high vapor pressure and high toxicity pose a much greater risk of exposure, and hence are generally not handled as reagents at our testing facility. A material which reacts slowly with water is much harder to destroy. Furthermore, for some highly toxic materials, respirator cartridges have unknown sorbent effectiveness. The thermal hazards testing group can perform on-site testing services in VSP2/ARSST equipment in these kinds of situations. Examples of such reagents include phosgene, hydrogen cyanide, and methyl isocyanate. While hydrogen cyanide can be scrubbed with sodium hydroxide, its high vapor pressure makes a lethal exposure easy to achieve in the event of a leak during handling. On-site testing services have been successfully provided at client facilities having the appropriate PPE (including supplied air respirators) and procedures for handling HCN and methyl isocyanate as reagents.
We can, however, perform tests that produce HCN, as it can be scrubbed with sodium hydroxide. In this case, the use of supplied air respirators and dedicated lab space for the clean-up necessitate premium pricing for these tests, but still less than going on-site.
High molecular weight diisocyanates, on the other hand, can be handled at our facilities owing to very low volatility. Although the short term exposure limit for toluene diisocyanate, for example is, 0.02 PPM, the low vapor pressure coupled with the use of a fume hood permits this material to be handled in our facilities. When handled at room temperature, conventional full facepiece cartridge respirators can be used. Great care must be taken to avoid skin exposure of these materials because they are strong skin and respiratory sensitizers and they have a slow reaction rate with water. Decontamination of tools/surfaces is done using an overnight soak in an aqeuous baking soda solution. Thermal decomposition and the reaction with water produces CO2 and by-products which are expected to be less toxic than the original diisocyanate. (Experience has shown these reactions tend to be foamy in nature.) Combustion, however is expected to produce HCN which must be appropriately scrubbed.
Volatile carcinogenic reagents such as ethylene oxide, 1,3-butadiene, and vinyl chloride can be handled at our facilities. In the case of ethylene oxide, OSHA mandates the use of a supplied air respirator. These materials are handled in generally small quantities with appropriate equipment to handle the high vapor pressure. In the case of 1,3-butadiene, the inhibitor is removed by passing the 1,3-butadiene through a column of alumina and then condensing the uninhibited monomer in a dry ice and acetone bath. The cold temperature permits the appropriate amount to be quickly weighed out using a syringe.
Case study of Dimethyl Sulfoxide (DMSO) Decomposition
When we started conducting tests involving dimethyl sulfoxide in the mid 1990s, the available MSDS listed the decomposition products as sulfur dioxide, methyl mercaptan, and formaldehyde. Thus one would expect non-condensable gas to be generated. In closed system VSP2 tests, we observed decompositions beginning in the 200°C to 240°C range. The temperature rise rates typically approach 100°C/min with associated pressure rise rates at 300°C of around 1000 psi/min in a closed VSP2 test cell. Owing to the small headspace volume (~30 ml) and the continued increase in temperature and pressure, the test cell ruptures since the standard containment pressure is limited to 1300 psi for safe operation. (The containment vessel is isolated from the nitrogen source when the pressure reaches 1300 psi and the test cell is then allowed to rupture). Upon cool-down the gas in the containment vessel must be scrubbed to treat the gases.
Using the MSDS listed products as a guide for post-test scrubbing, the sulfur dioxide can be handled using sodium hydroxide solution. To handle the strong stench of methyl mercaptan, a proprietary solution called Epoleon N-100 is used. (This material can be purchased through suppliers like McMaster Carr, where it is listed as a cutting fluid and coolant deodorizer.) To accommodate both of these sulfur compounds in a single stage scrubber, we make a weight percent sodium hydroxide in water. Although it would be ideal to scrub in multiple stages, we typically use a single stage and pass the gas (using a metering type valve) through a Pyrex® tube with a 40-60 micron frit on the end. Since VSP2 testing typically ends at an elevated pressure, a prohibitively long scrubbing time would be required using multiple stages. Owing to the smell and nature of the decomposition products, on-site VSP2 testing has been conducted when the client requires multiple tests in a campaign.
Recently in the course of testing (at FAI facilities) an employee asked how we knew we were safe in our scrubbing procedures and PPE used in handling DMSO decomposition products. An available 1993 memorandum from Ciba-Geigy, Ltd. dealing with hazards of DMSO provided the mechanism of the decomposition reaction with the associated products(1).
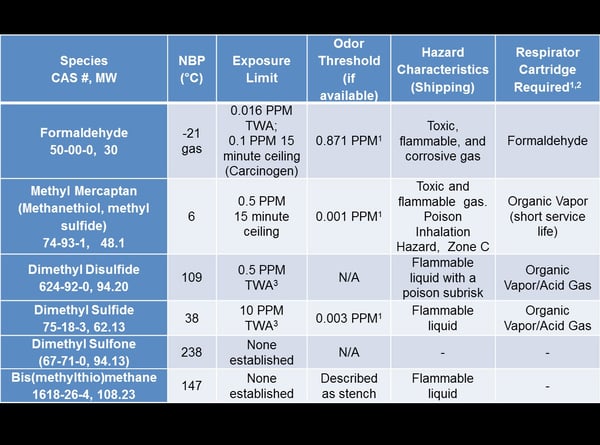
Table 1 provides some of the properties of the expected DMSO decomposition products. Conducting test operations in a properly functioning fume hood and venting the gasses slowly through an Epoleon N-100/caustic scrubbing solution mitigates the methyl mercaptan and sulfur dioxide. Therefore, the concentration of gases in the fume hood should be very low. Typically containment vessels are purged with nitrogen after the gases have been initially scrubbed. By following these operating procedures, we use full facepiece respirators equipped with multicontaminant cartridges. Furthermore, the other listed decomposition products are liquids for which the 3M Respirator Guide recommends organic vapor/acid gas cartridges. By replacing the cartridges twice during the workday (e.g. in the morning and afternoon), the “short service life” pertaining to methyl mercaptan is accommodated. The use of full facepiece cartridge respirators versus supplied air respirators facilitates test operator mobility and efficiency.
References:
(1) “Potential Hazards Involved in the Handling and Use of Dimethyl Sulfoxide (DMSO ),” Ciba-Geigy Ltd., Corporate Safety and Environment, Information B31, Basle, April 1993
For more information or to discuss process safety testing and engineering, please contact Charlie Askonas at Askonas@fauske.com, 630-323-8750

