James P. Burelbach, PhD, Fauske & Associates
INTRODUCTION
 In the chemical process industry bench-scale thermal hazard testing is an effective approach to quickly collect critical safety data for process scale-up and management of change.
In the chemical process industry bench-scale thermal hazard testing is an effective approach to quickly collect critical safety data for process scale-up and management of change.
These accepted test methods are directly relevant to the packaging, transport, and storage of radioactive waste that is or can become chemically reactive.
For chemically reactive waste streams it is vital to identify safe temperature and pressure conditions and to quantify adiabatic heat and gas generation rates in order prevent or accommodate thermal instability within the waste package or storage facility.
ORGANIC-NITRATE EXPERIMENTS (ARSST)
High level waste streams can include organic-bearing sludge and salt cake waste. Organic complexants like sodium acetate along with oxidizers like sodium nitrate present the potential for spontaneous runaway chemical reactions (thermal instability).
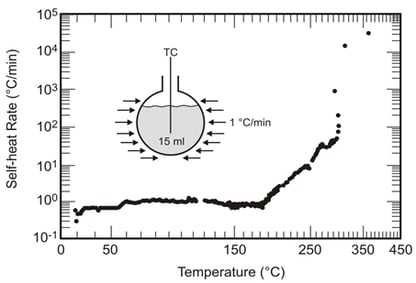
The data below are for 20.5% sodium acetate (6% Total Organic Carbon, or TOC) in a simulant oxidizer mixture heated at 1 °C/min. These data show significant exothermic activity at 200 °C with “ignition” at 300 °C.

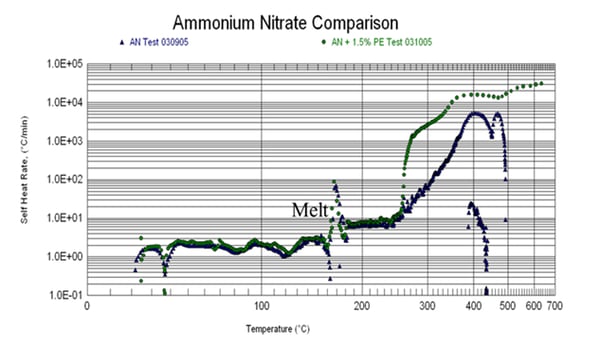
Ammonium nitrate is another common oxidizer that has been involved in large scale industrial disasters and is potentially explosive when mixed with organic fuel. The data below illustrate the effect of a small amount of organic contamination (polyethylene scrap).
ADIABATIC CALORIMETRY TOOLS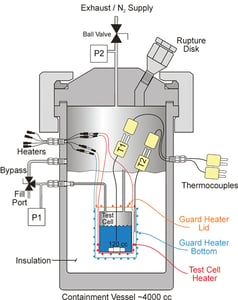
VSP2™ (Vent Sizing Package 2)
- Up to 100 ml sample size
- Stainless, Hastelloy, or glass-lined test cells
- Pressure balancing
- Closed cell testing gives direct vapor pressure
- Strong mixing suitable for two-phase or slurries
- Scale suitable for contamination studies
- Scale suitable for in-test dosing or sampling
- Test setup takes about 2 hrs
COMMON FEATURES
- Test solids or liquids (magnetic stirring)
- Light-weight test cell à low thermal inertia
- Test cell heat capacity << sample heat capacity
- “Phi-factor” ϕ close to unity ~ 1.05 to 1.1

- Evolved heat is not lost to the container (or the environment) but increases the sample temperature
COMMON BENEFITS
- Data are directly scalable
- Measure adiabatic temperature rise (ATR)
- Measure temperature rise rate dT/dt = f(T)
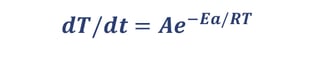
- Measure pressure rise rate dP/dt = f(T)
- Infer molar gas generation rate
- Kinetic modeling
COMMON APPLICATIONS
- Emergency vent sizing (pressure relief)
- Safe storage temperature
- Safe package/container size
- Time to maximum rate (TMR)
- Temperature of no return (TNR)
- Self-accelerating decomposition temperature (SADT)
ARSST™ (Advanced Reactive System Screening Tool)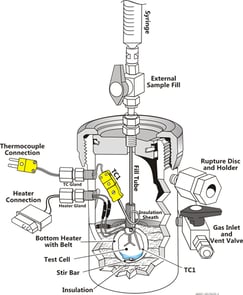
- Up to 10 ml sample size
- Scale suitable for thermal screening (identify energetic reactions)
- Normally open cell testing
- Scale suitable for large rates of gas generation (decomposition)
- Scale suitable for operation in a glove box or hot cell
- Test setup takes about 20 min
“RED OIL” EXPERIMENTS (VSP2)
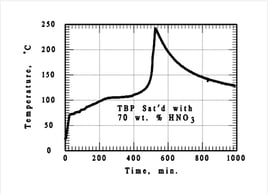
Tri-n-butyl phosphate (TBP) saturated with concentrated nitric acid (HNO3) can form two-layer organic/aqueous morphology in solvent extraction system evaporators and tanks. The organic phase reacts exothermically and under certain conditions this can lead to a thermal runaway
(e.g. Tomsk-7 reprocessing plant explosion in Russia, 1993).

CONCLUSIONS
ORGANIC-NITRATE EXPERIMENTS
- Organic contamination in an oxidizer such as sodium nitrate or ammonium nitrate can lead to an Arrhenius runaway reaction followed by a wave-like “propagating reaction”
- Thermal hazard testing can identify the minimum TOC required for a reaction to propagate, and the observed kinetics provide a technical basis for safe packaging and storage
RED OIL EXPERIMENTS
Two separate exotherms were observed
- The 1st exotherm at 80 °C is mild (< 1 °C/min)

- Tempering (under atmospheric pressure) occurs at about 100 °C due to evaporation of dissolved water
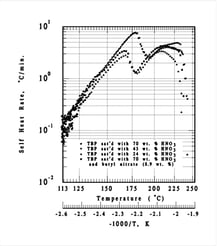
- The 2nd exotherm (two peaks) is stronger (< 10 °C/min)
- Peak rates are reduced for lower HNO3 concentrations or in the presence of decomposition products (e.g. butyl nitrate) but the activation energy (inferred from the dT/dt slope) is unchanged
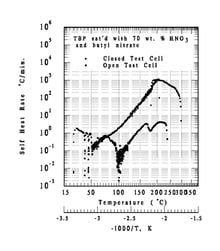
- In a “closed cell” configuration (e.g. if the process vessel vent is closed or plugged) tempering does not occur and pressure builds
- Closed cell self-heat rates increase exponentially to 1000 °C/min
- The Tomsk-7 accident can be explained by a combination of weak tempering and insufficient venting capacity

REFERENCES
Burelbach & Theis (2005). “Thermal Hazards Evaluation using the ARSST,” 3rd Intl. Symposium On Runaway Reactions, Pressure Relief Design, and Effluent Handling, Cincinnati.
Epstein, M., et al. (2008). “Thermal Stability and Safe Venting of the Tri-n-Butyl Phosphate-Nitric Acid-Water (‘Red Oil’) System—II: Experimental Data on Reaction Self-Heat Rates and Gas Production and their Correlation,” Vol. 163, Nuclear Technology, Aug. Ibid, “III: Predictions of Thermal Stability Boundaries and Required Vent Size.”

